-
E-MAIL
uscert@naver.com

-
E-MAIL
uscert@naver.com

-
TEL
02-529-8005

-
TEL
02-6226-9776


全球 RA 许可证
 HOME > 全球 RA 许可证 > 韓国
HOME > 全球 RA 许可证 > 韓国
韓国
Definition of In Vitro Diagnostic Medical Device in Korea (MFDS)
Reagents, control and correction materials, instruments, machinery, equipment, software, etc. used alone or in combination to examine samples derived from humans or animals in vitro. A product that falls under any of the following items as a medical device under Article 2 (1) of the Medical Devices Act (In Vitro Diagnostic Medical Devices Act Article 2)
- A. Products used for the purpose of diagnosing physiological or pathological conditions
- B. Products used for the purpose of determining the predisposition of a disease or observing the prognosis of a disease
- C. Products used for the purpose of providing information on congenital disorders
- D. Products used for the purpose of providing information necessary to determine safety and suitability when transfusion or transplantation of blood or tissue to another person
- E. Products used for the purpose of predicting treatment response and treatment outcome
- F. Products used to determine treatment options or to monitor the effectiveness or side effects of treatment
Scope of application of in vitro diagnostic medical devices
1. A person who intends to obtain a manufacturing/import license or manufacturing/import certification for in vitro diagnostic medical devices, or to file a manufacturing/import report;
2. A person who intends to manufacture or import an in vitro diagnostic medical device for clinical performance testing
3. A manufacturer or importer who intends to undergo conformity certification and regular inspection (examination for conformity certification, etc.)
[In vitro diagnostic medical device manufacturing and quality control standards (No. 2020-33) Article 3]
• In vitro diagnostic medical devices are classified into four classes according to their intended use and the degree of potential hazards to personal and public health.
* Regulations on in vitro diagnostic medical device items and grades by item (Notice No. 2020-34)
Class 1 - Reagents with low potential risk to individual and public health, devices used for legal diagnostic purposes
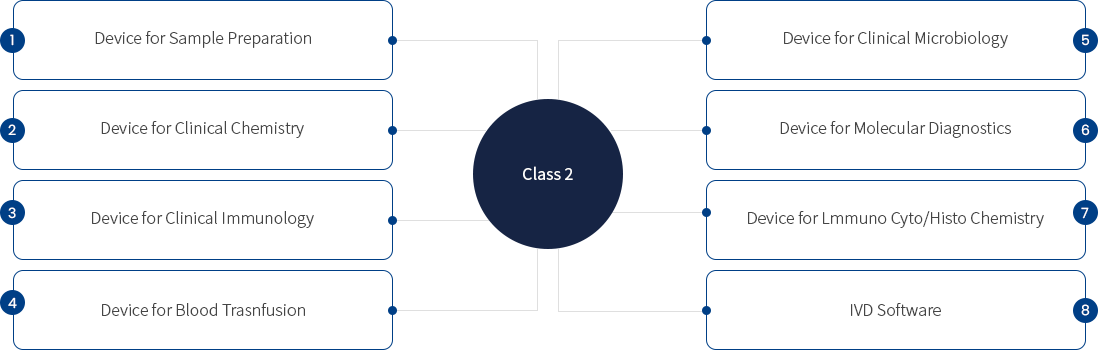
Class 2 - Devices with a moderate potential risk to individuals but a low potential risk to public health
Class-3 - Devices used for diagnosis, treatment, disease staging, and tests that have a decisive effect on treatment
Class-4 - Equipment used for screening donors for blood transfusions or transplants to others or for high personal risk.
In vitro diagnostic medical device category
• Receives GMP conformity recognition for each item group, and additional examination is required when adding item groups
Medical device licensing procedure as defined by Korea (MFDS)
• Class 1
Item report (Korea Medical Device Safety Information Service, NIDS)
- 1) Registration of item report application [In vitro diagnostic medical device electronic civil complaint window] → Accept the report after registration is complete
- 2) Technical documentation includes: Name (product name, item name, model name) / Classification number (grade) / Shape and Structure / Purpose of use / How to use / Precautions for use / Manufacturer (in case of import or consignment of manufacturing process) / Same product license number
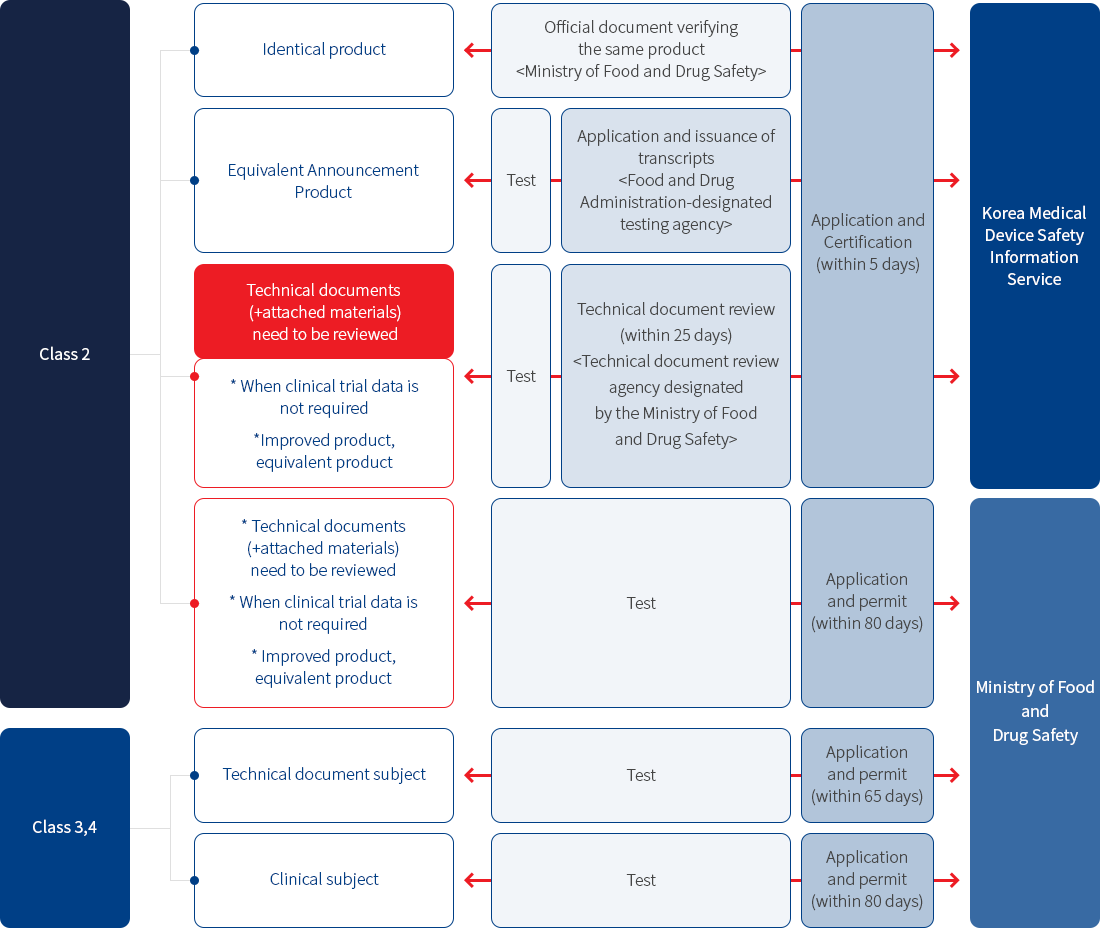
• Class 2
Approval of the item (processed by the Ministry of Food and Drug Safety)
- 1) Item classification criteria
- 2) Classification of equivalent products: purpose of use, principle of action, raw material, performance
- 3) New product: Medical devices that are not identical in purpose of use and principle of action to in vitro diagnostic medical devices that have already been approved
- 4) Improved product: An in vitro diagnostic medical device that has the same purpose and principle of action as that of an in vitro diagnostic medical device that has already been approved, but does not have the same raw materials or performance
- 5) Equivalent product: In vitro diagnostic medical device that has the same purpose, principle of action, raw material and performance as the in vitro diagnostic medical device that has already been approved
• Class 3 & 4
Approval of items (inclusive review and proceeding by the Ministry of Food and Drug Safety Headquarters)
- 1) Same flow as class 2. The technical document review period is 65 days and 80 days for collective application for permission, so there is a difference in the period.
-
GMP in Korea (MFDS)
Medical device GMP specifies the requirements of the quality management system applied to the design, development, production, installation and service of medical devices. Detailed GMP requirements can be found in 「Notice on Medical Device Manufacturing and Quality Control Standards」. It applies to all medical device manufacturers and importers, the type of examination is divided into document examination and on-site examination. After the initial review, you must undergo a regular renewal evaluation every three years. If additional items other than previously certified items occur among the item groups classified by GMP, or if the location is changed, additional on-site evaluation may be required.

 韩语
韩语  英语
英语  日语
日语  中文
中文