-
E-MAIL
uscert@naver.com

-
E-MAIL
uscert@naver.com

-
TEL
02-529-8005

-
TEL
02-6226-9776


全球 RA 许可证
 HOME > 全球 RA 许可证 > 中国
HOME > 全球 RA 许可证 > 中国
中国
What is cosmetics as defined in China (CFDA (NMPA))
Chemical industrial products or sophisticated chemical products intended to keep all parts of the body surface (skin, hair, nails, lips, teeth, etc.) clean, nourishing, beautifying, decorating or changing appearance, and removing body odors by applying or spraying on the body or other similar methods.
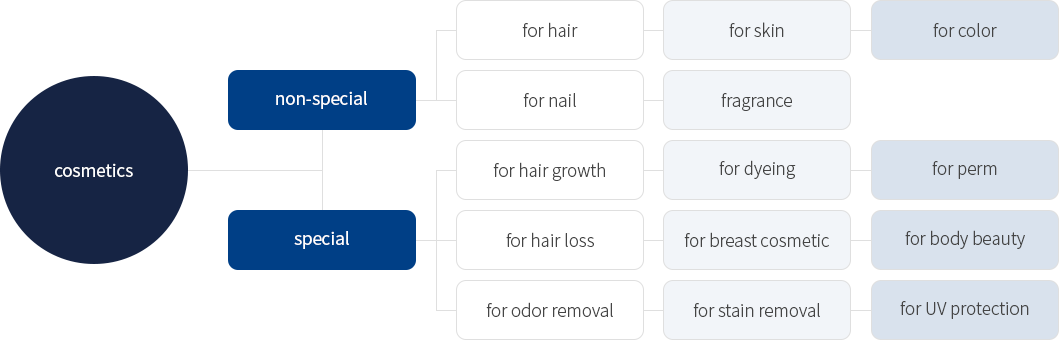
• CFDA (NMPA) Cosmetic Classification
• Cosmetics sub-category
| division | classification | subcategory | Related products |
|---|---|---|---|
| cosmetics | non-special | for hair | hair lotion, hair oil, etc. |
| for skin | Skin, lotion, cream, oil, etc. | ||
| for color | shadows, lipsticks, etc. | ||
| for nail | manicure, etc. | ||
| fragrance | Perfume, fragrance, etc. | ||
| special | for hair | Hair growth agent, hair loss prevention agent, etc. | |
| for dyeing | Dye, bleach, bleach, etc. | ||
| for perm | Perm, etc. | ||
| for hair loss | Depilatory agent, abrasive agent, etc. | ||
| for breast cosmetic | Breast beauty cosmetics, etc | ||
| for body beauty | dry cosmetics, etc. | ||
| for odor removal | Sweat remover, odor remover, etc. | ||
| for stain removal | stain remover, etc. | ||
| for UV protection | Sun oil, sunscreen, etc. |
Registration procedure for exporting cosmetics to China (CFDA (NMPA))
① Prior review
② Selection of responsible company
③ Authorization of responsible company (ID/PW issuance)
④ Review ingredients and labels
⑤ Product testing (about 2 months for non-specialty types / about 4-6 months for special types)
⑥ CFDA (NMPA) permit application
⑦ CFDA (NMPA) review
⑧ CFDA (NMPA) final evaluation
⑨ Issuance of sanitary permit
※ Responsible company designation required when registering cosmetics in China
Documents to be submitted for export of cosmetics to China (CFDA (NMPA))
| No. | List of documents to be submitted |
|---|---|
| 1 | Product name (Korean/Chinese/English), company name (Korean/Chinese/English), address |
| 2 | Notarized copy of the responsible company |
| 3 | product ingredient list |
| 4 | Product mass specification |
| 5 | Product label content (Korean/Chinese) |
| 6 | Product sample (quantity can be determined depending on product capacity, customs fees may incur) |
| 7 | Manufacturing Sales Certificate |
| 8 | Manufacturing process flow chart |
| 9 | Consignment processing agreement (in case of consignment processing) |
| 10 | Original ISO or GMP certificate (when processing on consignment) |
| 11 | Scientific literature data based on use for functionality (applicable to special classes) |
| 12 | Scientific literature data based on use for functionality (applicable to special classes) |
Composition of the cosmetic ingredient list required by China (CFDA (NMPA))
Name of all ingredients, purpose of use, mixing ratio (in order of highest)
Ingredient names are based on the INCI name (if there is no INCI name, a chemical dictionary is available)
Colorants provide CI number of [Cosmetics Hygiene Standards]
When using animals, plants, microorganisms, minerals, etc. as raw materials, Latin names are provided
Animal organ extracts provide proof of source of raw materials, quality standards, and permission for use of raw materials in the country of origin
For products that consist of multiple products (dye, perm), the ingredient list must be submitted separately
If there are multiple restricted substances, specify the ratio of each substance
If there is an ingredient that meets the standard requirements for restricted substances in [Cosmetics Sanitary Standards], submit the quality standard certification from the raw material supplier
Criteria for determining whether cosmetics are cosmetic as defined by China (CFDA (NMPA))
| Products classified as cosmetics | Products not classified as cosmetics |
|---|---|
| How to use Products that are applied or sprayed Products that come into contact with the surface of the human body | Products that achieve a cosmetic purpose by taking or injecting |
| Products intended for use in cleanliness, odor removal, moisturizing, beauty and decoration | Products that come in contact with the oral cavity or mucous membranes Products with functions such as prevention or treatment of diseases |
Test items for export of cosmetics to China (CFDA (NMPA))
Sanitary chemical test
Acute oral toxicity test
Microbial test
Acute skin irritation test
Primary eye stimulation test
Skin phototoxicity test
Skin transformation test
pH measurement
Multilateral skin irritation test
Mouse typhus salmonella back mutation test
In vitro mammalian cell chromosomal abnormality test
Prohibited and restricted substances measurement test (including measurement of UV absorbers, hormones, dye products restricted substances and peracids)
Human safety and function test

 韩语
韩语  英语
英语  日语
日语  中文
中文